|
|
Build Your Online Product Catalogs?
| Product Name: |
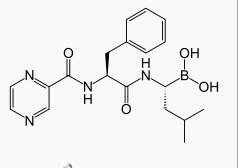
Bortezomib
|
| Supply Ability: |
|
| Related proudcts |
d-xylose, bortezomib, lincomycin hcl, |
| Specifications |
Enterprise standard |
| Price Term: |
CNF, CIF |
| Port of loading: |
|
| Minimum Order |
|
| Unit Price: |
|
|
| Bortezomib was originally synthesized in 1995 (MG-341) at a co***ny called Myogenics, which soon changed its name to ProScript. After promising preclinical results, the drug (PS-341) was tested in a small Phase I clinical trial on patients with multiple myeloma cancer. ProScript ran out of money and was bought by Leukosite in May 1999. Leukosite in turn was bought by Millennium Pharmaceuticals in October 1999. At this point in time, the project had low priority amongst other projects at the co***ny. This changed significantly when one of the first volunteers to receive the drug in the clinical trial achieved a complete response and was still alive four years later. At the time this was a remarkable result. Later clinical experimentation indicates the possibility of a complete response in 15% of patients in a similar condition, when treated with bortezomib. In May 2003, seven years after the initial synthesis, bortezomib (Velcade) was approved in the United States by the Food and Drug Administration (FDA) for use in multiple myeloma, based on the results from the SUMMIT Phase II trial.[2] Another commercially available bortezomib product - Bortenat (Natco Pharma, India), reportedly contains substantially more active entity than declared, potentially and even more resulting in increase toxicity. Moreover, Bortenat has some other chemical and formulation deviations from the registered ethic product Velcade (Millennium Pharmaceuticals and Janssen-Cilag), with unclear clinical i***ct.[3] |
| Company: |
Ningbo Highmomedical Technology Co.,Ltd
|
| Contact: |
Mr. Chen Haitao |
| Address: |
No.19 TianRong Street, Daxing Bio-medicine Industry Park, Zhongguancun Science Park , Beijing ,China |
| Postcode: |
315000 |
| Tel: |
86-15867886276 |
| Fax: |
86-574-87297028 |
| E-mail: |

|
|
|
|